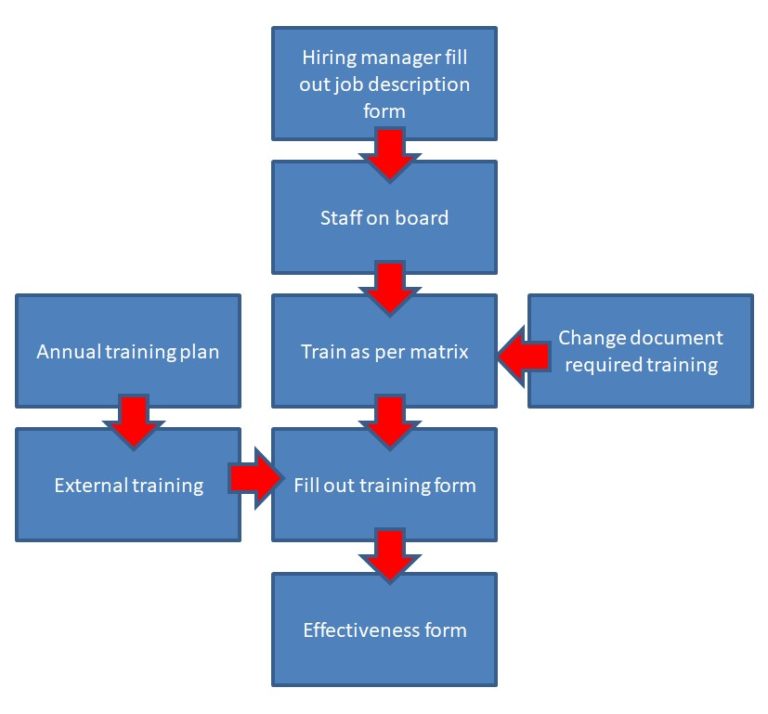
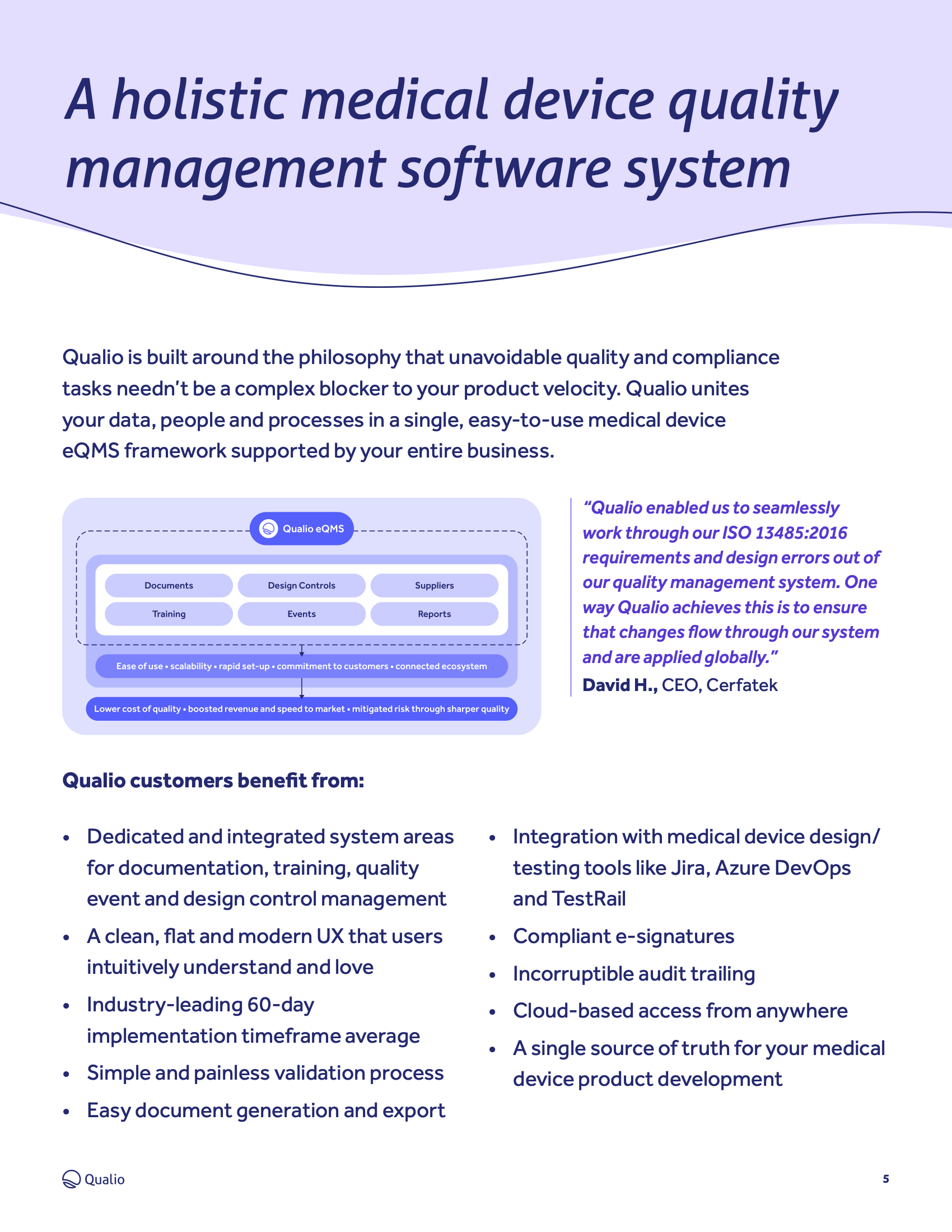
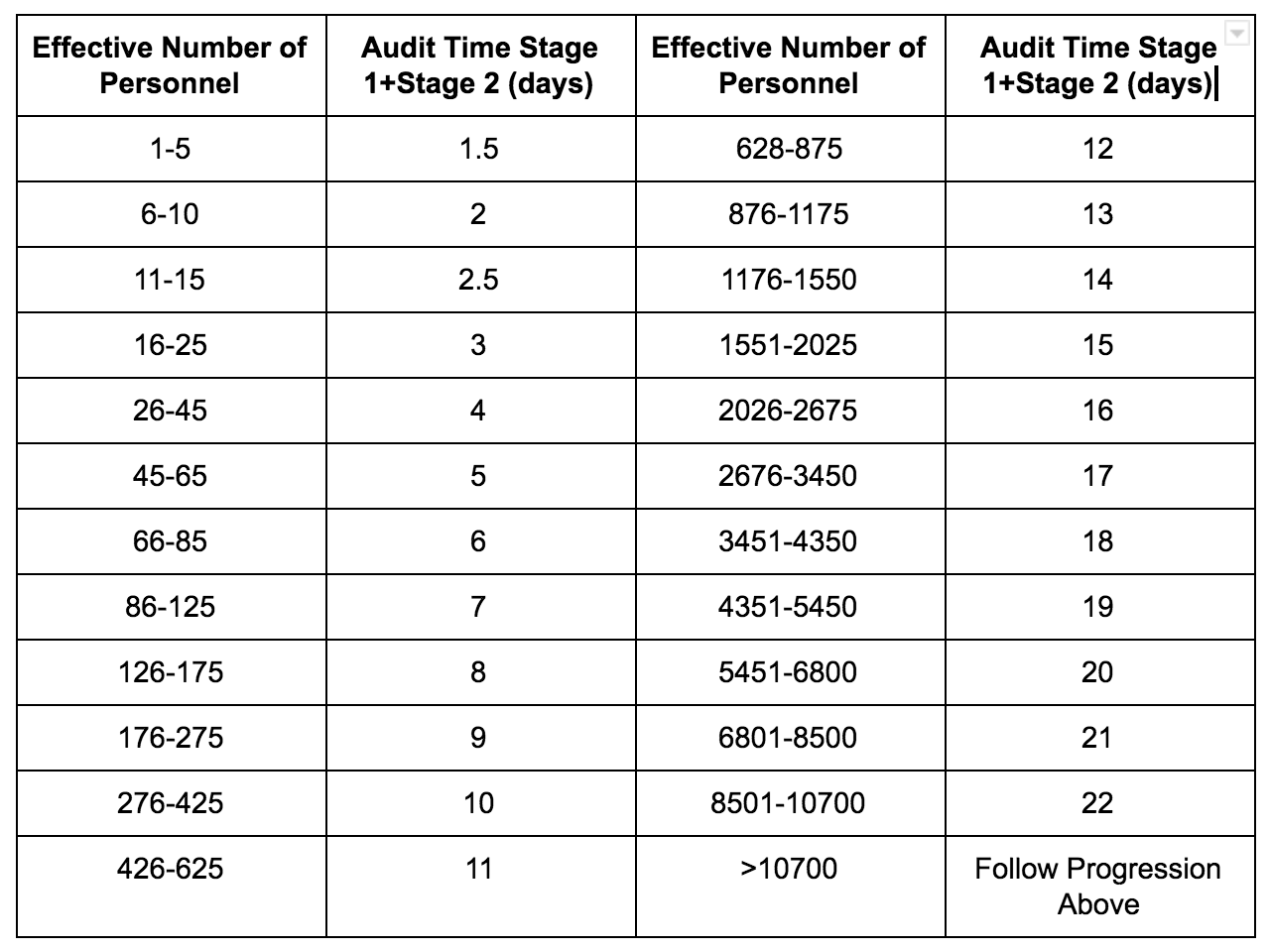
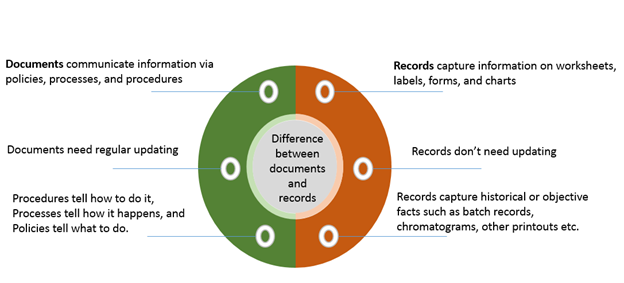
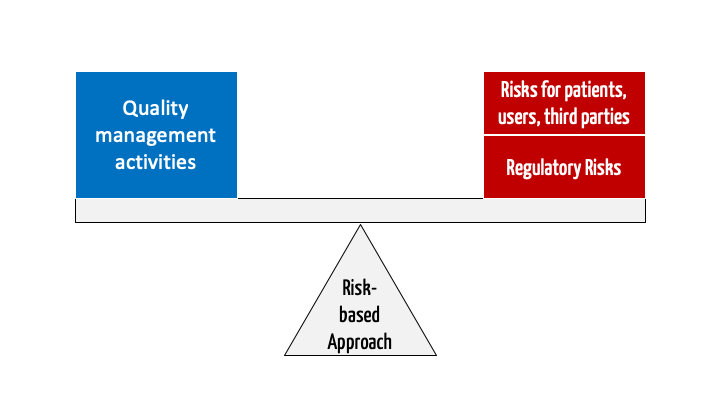
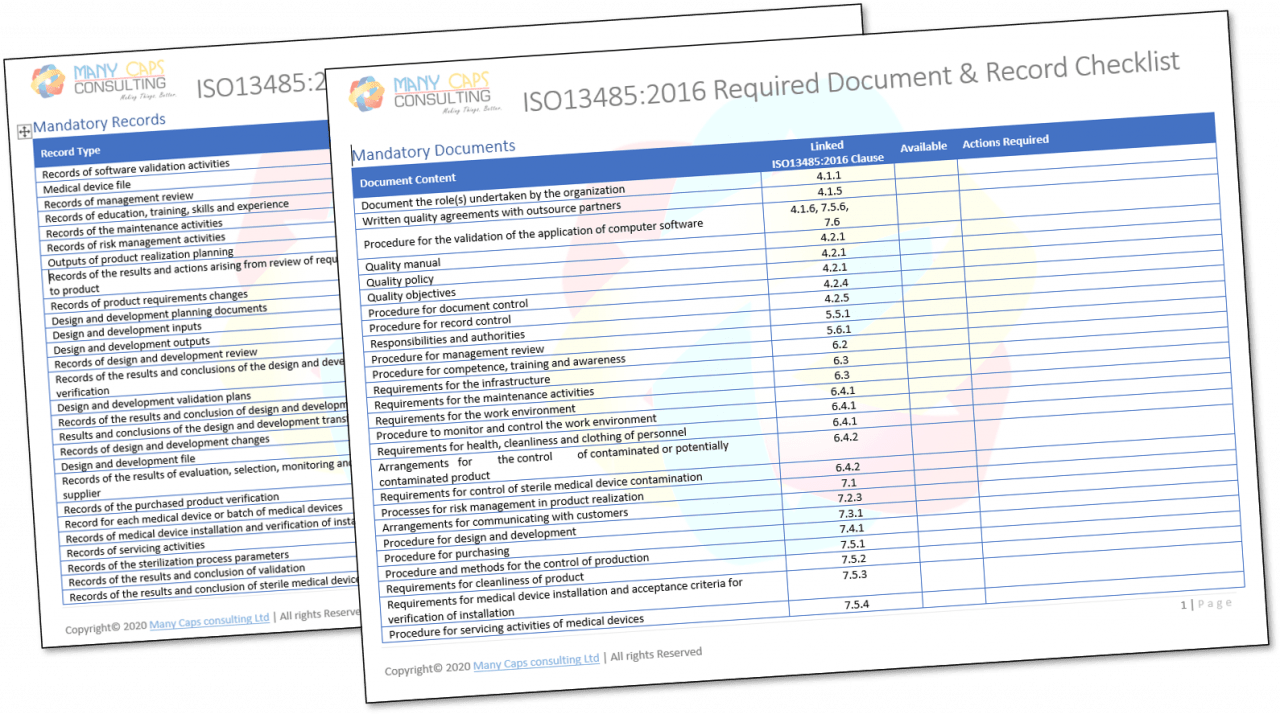
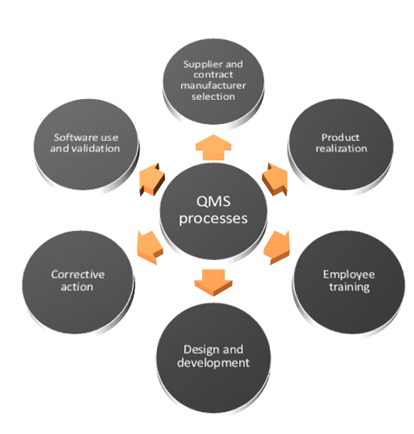
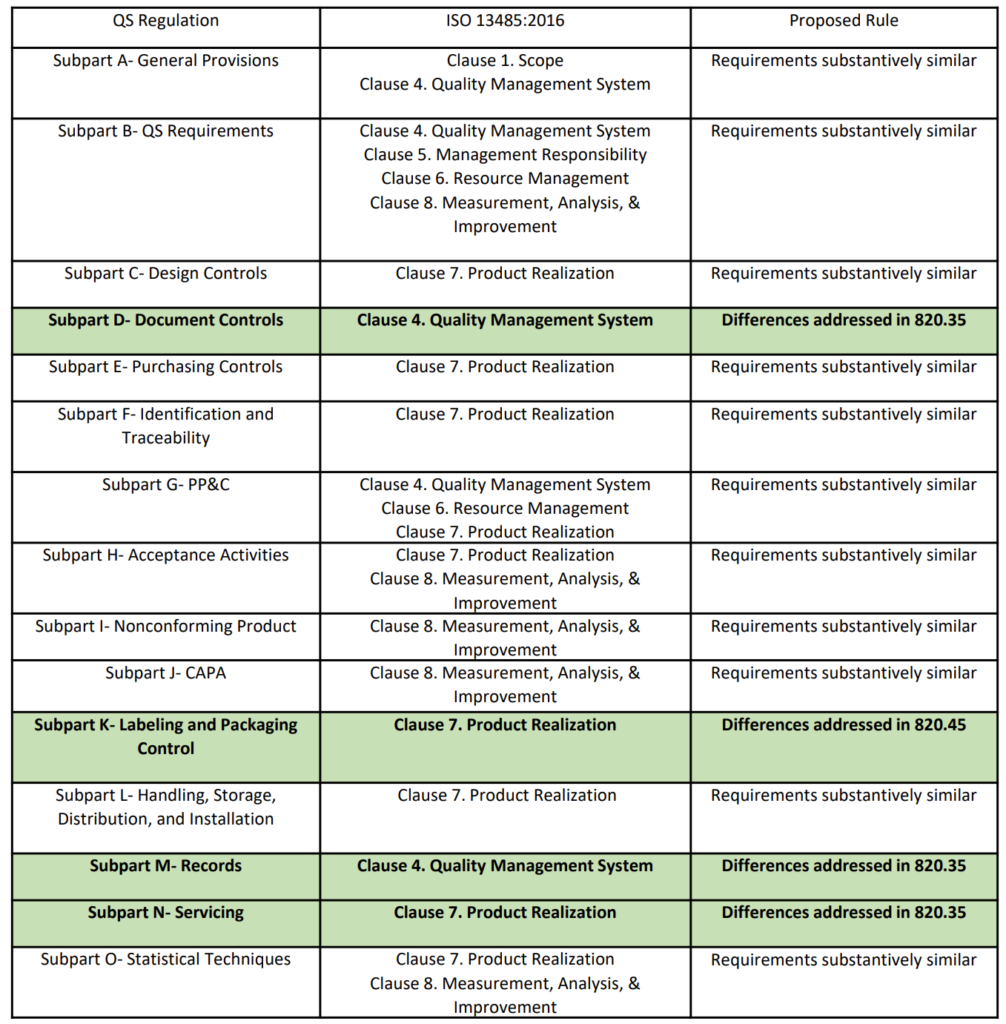
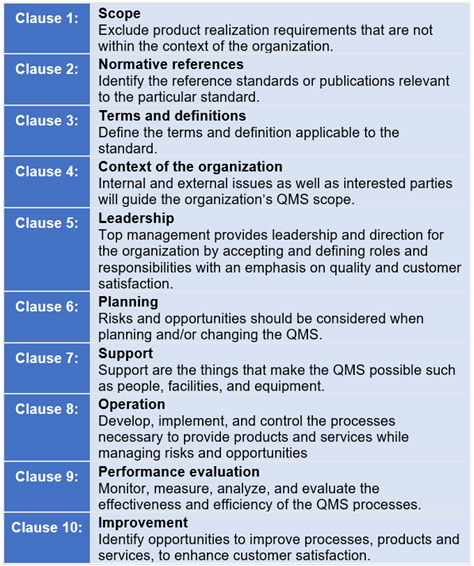
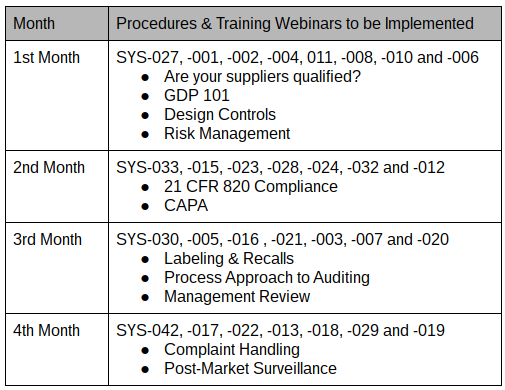
Best Tips: ISO 13485 procedures with our free template (Version 2016) - Medical Device Regulation and ISO quality standard

Poor Quality of ISO Documents | Cost of Quality Calculation Example | ISO 9001 Quality System - YouTube

ISO13485 Quality management systems (QMS) Standards for Medical Device – ISO Templates and Documents Download